Você sabia?
Você pode clicar duas vezes em uma palavra para procurá-la na TermGallery.
Você pode clicar duas vezes em uma palavra para procurá-la na TermGallery.
Significados de polar molecule em inglês
russo
полярные вещества espanhol
molecula polar 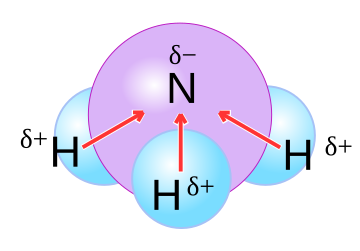
Molecule, interact through dipole–dipole intermolecular forces and hydrogen bonds, must contain polar bonds due to a difference in electronegativity between the bonded atoms.
Veja maisUso de polar molecule em inglês
1
That's because water is a polar molecule, while grease or oil is a nonpolar molecule.
2
Water does this because it is a polar molecule.
3
Such an asymmetric bilayer is a highly effective barrier for polar molecules.
4
Polar molecules like water attract each other like magnets, too.
5
The polar molecules again try to orient themselves in sympathy with the fast undulations of the field.
6
A hydrogen bond results from the attraction between oppositely charged ends of polar molecules (or portions of molecules).
7
Multiple assays are used to show that FliP suffices to form a channel that can conduct a variety of medium-sized, polar molecules.
8
Temperature-dependent molecular desorptions for six different polar molecules were measured in real-time to study the desorption kinetics and extract the binding affinities.
9
The water molecules are polar and form strong intermolecular bonds with other polar molecules, which are able to balance out their electrical charges.
10
Furthermore, the heterodyne sensing technique enables electrical probing and tuning of the noncovalent physisorption of polar molecules on graphene surface for the first time.
Esta colocação é formada por:
Translations for polar molecule
espanhol

